Abstract
Background: In the pivotal ZUMA-5 (Z-5) trial, axicabtagene ciloleucel (axi-cel; an autologous anti-CD19 chimeric antigen receptor T-cell therapy) demonstrated high rates of durable response in r/r FL patients, including those with high-risk disease such as patients who progressed within 24 months of initiating first-line chemoimmunotherapy (POD24).
Aims: To compare clinical outcomes from updated 24-month Z-5 to a weighted sample from the international SCHOLAR-5 (S-5) external control cohort.
Methods: The international S-5 cohort data were extracted for r/r FL patients from 7 institutions in 5 countries who initiated a third or higher (3L+) line of therapy (LOT) after July 2014. Data from the pivotal idelalisib DELTA trial was also included in the S-5 cohort. Z-5 trial eligibility criteria were applied to the S-5 cohort, with patients excluded or censored upon transformation. The S-5 and Z-5 cohorts were balanced for patient characteristics through propensity scoring on prespecified prognostic factors and standardized mortality ratio weighting. Characteristics with a standardized mean difference (SMD) <0.1 were deemed balanced. Overall response rate (ORR) was compared using odds ratio. Overall survival (OS), progression-free survival (PFS) and next treatment-free survival (NTFS) were evaluated using Kaplan-Meier analysis. Subgroup analysis was conducted on patients who initiated a fourth or higher (4L+) LOT.
Results: 143 patients were identified in S-5, reducing to 85 patients after applying propensity score weights, versus 86 patients in Z-5. Median follow-up time for Z-5 and S-5 were 29.4 and 26.2 months respectively. Variables that were successfully balanced (SMD<0.1) included POD24, number of prior LOT, relapsed vs refractory, prior stem cell transplant, size of largest nodal mass, response to prior LOT, time since last therapy and age (Table 1). Despite weighting, the S-5 cohort had a higher proportion of ECOG 1 vs 0 (66.9% vs 40.7%) at baseline.
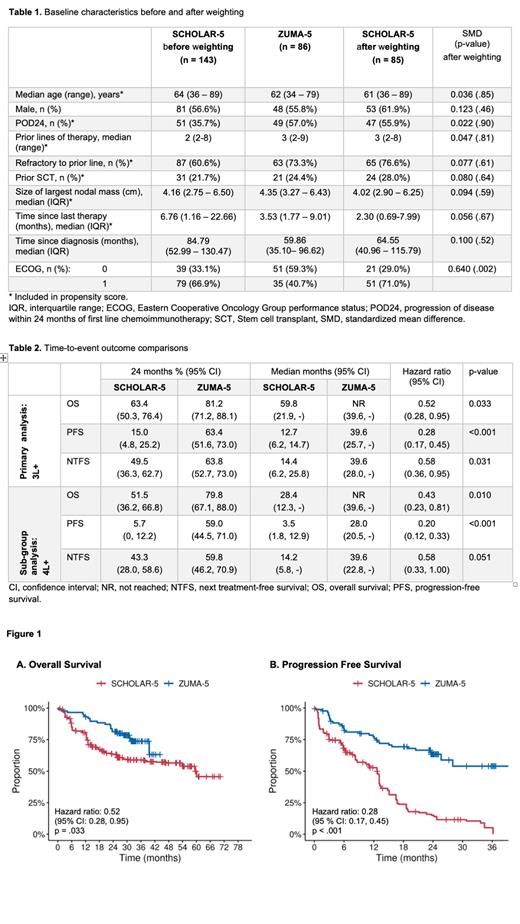
In 3L+ patients, the ORR was 42/85 (49.9%) in S-5 compared to 81/86 (94.2%) in Z-5 for an odds ratio of 16.2 (95% confidence interval [CI]: 5.6-46.9). The median OS was not reached in Z-5 while median PFS was 39.6 months. In S-5 median OS and PFS were 59.8 months and 12.7 months, respectively (Table 2). The hazard ratios for OS and PFS were 0.52 (95%CI: 0.28-0.95) and 0.28 (0.17-0.45) (Figure 1). In sub-group analysis of 4L+ patients, which compared 60 patients from Z-5 to 59 patients from S-5, improvements in OS and PFS outcomes were more pronounced (Table 2).
Summary/Conclusion: Compared to currently available therapies in r/r FL patients, axi-cel demonstrated a substantial and statistically significant improvement in meaningful clinical endpoints including ORR, PFS, NTFS and OS, highlighting the durable treatment effect of axi-cel. These findings suggest that axi-cel addresses an important unmet medical need for r/r FL patients.
Palomba: PCYC: Consultancy; BeiGene: Consultancy; Juno: Patents & Royalties; Pluto: Honoraria; Wolters Kluwer: Patents & Royalties; Kite: Consultancy; Notch: Honoraria, Other: Stock; Seres: Honoraria, Other: Stock, Patents & Royalties, Research Funding; Priothera: Honoraria; Nektar: Honoraria; Lygenesis: Honoraria; Magenta: Honoraria; Rheos: Honoraria; WindMIL: Honoraria; Ceramedix: Honoraria; Novartis: Consultancy. Patel: Kite, A Gilead company: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company. Deighton: Delta Hat: Current Employment. Jacobson: Lonza: Consultancy, Honoraria, Other: Travel support; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support; AbbVie: Consultancy, Honoraria; Nkarta: Consultancy, Honoraria; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Other: Travel support; Precision Biosciences: Consultancy, Honoraria, Other: Travel support; Clinical Care Options: Speakers Bureau; Axis: Speakers Bureau; Humanigen: Consultancy, Honoraria, Other: Travel support; Pfizer: Consultancy, Honoraria, Other: Travel support, Research Funding; Celgene: Consultancy, Honoraria, Other: Travel support. Nahas: Kite: Current Employment; Gilead: Current equity holder in publicly-traded company. Jung: Kite, a Gilead Company: Current Employment; Amgen, Kura, Gilead, and Turning Point: Current equity holder in publicly-traded company. Hatswell: Delta Hat: Current Employment. Kanters: RainCity Analytics: Current Employment. Limbrick-Oldfield: RainCity Analytics: Current Employment. Wade: Kite, A Gilead Company: Consultancy; Amgen: Consultancy; Allergan: Consultancy. Thornton Snider: Kite, a Gilead Company: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Gilead: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Neelapu: Takeda Pharmaceuticals and related to cell therapy: Patents & Royalties; Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene Therapeutics, Cell Medica/Kuur, Incyte, Precision Biosciences, Legend Biotech, Adicet Bio, Calibr, Unum Therapeutics and Bluebird Bio: Honoraria; Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene, Kuur, Incyte, Precision BioSciences, Legend, Adicet Bio, Calibr, and Unum Therapeutics: Other: personal fees; Kite, a Gilead Company, Bristol Myers Squibb, Merck, Poseida, Cellectis, Celgene, Karus Therapeutics, Unum Therapeutics (Cogent Biosciences), Allogene, Precision BioSciences, Acerta and Adicet Bio: Research Funding. Gribben: Abbvie: Honoraria; AZ: Honoraria, Research Funding; BMS: Honoraria; Gilead/Kite: Honoraria; Janssen: Honoraria, Research Funding; Morphosys: Honoraria; Novartis: Honoraria; Takeda: Honoraria; TG Therapeutis: Honoraria. Radford: ADC Therapeutics: Consultancy, Current holder of individual stocks in a privately-held company, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Current holder of individual stocks in a privately-held company; BMS: Honoraria. Bobillo: Gilead: Speakers Bureau; F. Hoffmann-La Roche Ltd: Consultancy, Speakers Bureau. Ghesquieres: Gilead Science: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Roche: Consultancy; Mundipharma: Consultancy, Honoraria; Janssen: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal